ProContactVisio: Visualizing Protein Intramolecular Contacts
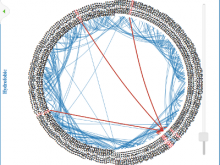
Predicting changes in protein function based on changes in sequence remains a significant challenge in biology, which is addressed in BioVis 2013 Data Contest. In this visualization, which is part of an effort to elucidate which mutations in protein sequence can impact on function, we tackle the problem of visualizing intramolecular contacts in proteins. The contacts among residues are very important to stabilize the tridimensional structure of a protein, which is cosely related to its function. Therefore, contacts can help to characterize the structure and they have been used to propose structural signature for proteins in many works. Intramolecular contacts were calculated at atomic level and mapped to residue level through a geometric criteria. For each protein, we generated a Voronoi diagram followed by its Delaunay tesselation and, based on Sobolev, atomic contacts were classified in hydrofobic, atractive, repulsive, hydrogen bond and aromatic. So, for each protein, we have five graphs, one for each type of contact, plus one general graph, which represents contacts without distinguish its type. Each type of contact can be further exploited by clicking on the main visualization. Visualizing different types of intramolecular contacts can provide insights on which residues could potencially lead to a mutation that impacts on function. If a residue which participates in many contacts is changed in sequence, it may disturb the structure, which is likely to cause a change in function.We are interested in nontrivial contacts, for example those between residues that are distant in sequence, so we excluded peptide bonds.